

Real life notes: If you end up using PCC in the lab, don’t forget to add molecular sieves or Celite or some other solid to the bottom of the flask, because otherwise you get a nasty brown tar that is a real major pain to clean up. The electrons from the C-H bond move to form the C-O bond, and in the process break the O-Cr bond, and Cr(VI) becomes Cr(IV) in the process (drawn here as O=Cr(OH)2 ). The C-O double bond is formed when a base removes the proton on the carbon adjacent to the oxygen. A chloride ion is then displaced, in a reaction reminiscent of a 1,2 elimination reaction, to form what is known as a chromate ester. Carbonyl function The carbonyl group is a super function because many common functional groups are based on a carbonyl, including: aldehydes, ketones, carboxylic acids, esters, amides, acyl. Secondly, a proton on the (now positive) OH is transferred to one of the oxygens of the chromium, possibly through the intermediacy of the pyridinium salt. Carboxylic acids can be shown in text as: RCOOH Carboxylic acids are weak Bronsted acids and they liberate CO2 from carbonates and hydrogen carbonates. You would normally use small quantities of everything heated in a test tube stood in a hot water bath for a couple of minutes. The first step is attack of oxygen on the chromium to form the Cr-O bond. Carboxylic acids and alcohols are often warmed together in the presence of a few drops of concentrated sulphuric acid in order to observe the smell of the esters formed. The elimination reaction can occur because we’re putting a good leaving group on the oxygen, namely the chromium, which will be displaced when the neighboring C-H bond is broken with a base. It is also used to oxidize the -methylene group adjacent to a carbonyl group to give a 1,2. We’re going from a carbon-oxygen single bond to a carbon-oxygen double bond. Selenium dioxide, SeO2 is an oxidizing agent generally employed in the allylic oxidation of alkenes to furnish allylic alcohols, which may be further oxidized to conjugated aldehydes or ketones. How does it work? Oxidation reactions of this sort are actually a kind of elimination reaction.
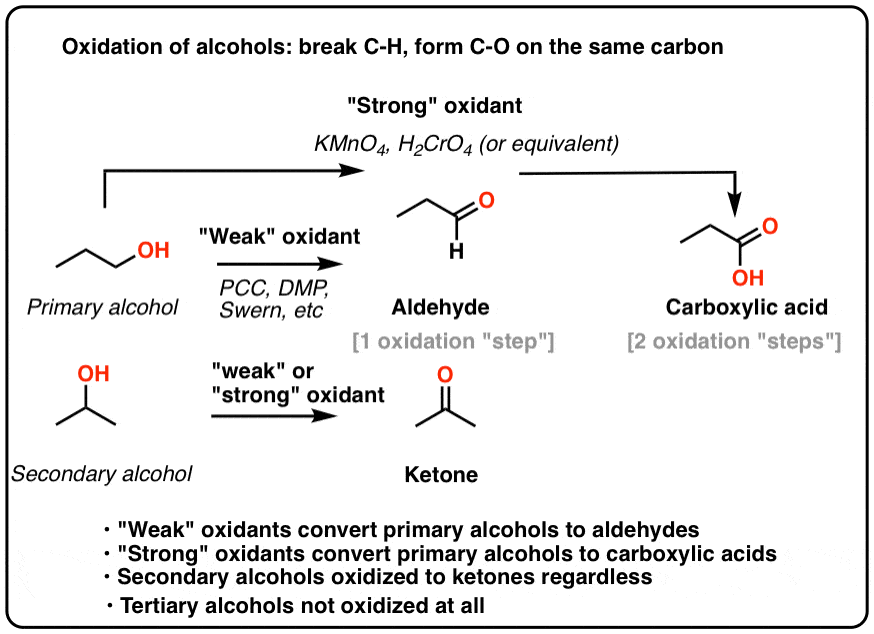
This is not a concern with ketones, since there is no H directly bonded to C. If water were present, it can ad to the aldehyde to make the hydrate, which could be further oxidized by a second equivalent of PCC were it present. One has to be careful with the amount of water present in the reaction.The byproducts (featured in grey) are Cr(IV) as well as pyridinium hydrochloride. If you add one equivalent of PCC to either of these alcohols, you obtain the oxidized version.Similar to or the same as: \(CrO_3\) and pyridine (the Collins reagent) will also oxidize primary alcohols to aldehydes. Pd/C along with NaBH4 in aqueous ethanol or methanol and either K2CO3 or KOH as base at room temperature under molecular oxygen or air is capable of oxidizing. Unlike chromic acid, PCC will not oxidize aldehydes to carboxylic acids.

6.1 Glucose Glucose -Oxidation with Bromine water> Gluconic acid HOOC-(CHON) 4 CH 2 OH 6. (general formula of HOOC-(CHOH) n CH 2 OH ). PCC oxidizes alcohols one rung up the oxidation ladder, from primary alcohols to aldehydes and from secondary alcohols to ketones. Aldonic acids or sugar acids are obtained upon the oxidation of aldehyde group of an aldose sugar to convert to carboxilic acid functional group i.e. The imidic acid is then deprotonated by the base and after another proton-transfer, the a mide intermediate is formed.Pyridinium chlorochromate (PCC) is a milder version of chromic acid. The base-catalyzed nitrile hydrolysis starts with a nucleophilic addition of the hydroxide ion to the C-N triple bond forming an intermediate with a negative charge on the nitrogen which quickly remove a proton transforming into an imidic: The Mechanism of Base-Catalyzed Nitrile Hydrolysis In the second part, the amide is hydrolyzed to a carboxylic acid by the mechanism we discussed earlier: The tautomerization mechanism can be shown in two proton-transfer steps starting with a protonation of the nitrogen: An imidic acid is the nitrogen analog of an enol and just like the keto-enol tautomerization, the more stable amide tautomer predominates the equilibrium: In the first step of acid-catalyzed hydrolysis, the protonation of the nitrogen activates the C-N triple bond for a nucleophilic attack of water:Īfter a deprotonation, a tautomer of an amide, called an imidic acid, is formed. The Mechanism of Acid-Catalyzed Nitrile Hydrolysis In both cases, the transformation consists of two main parts conversion of the nitrile to an amide and hydrolysis of the amide to the corresponding carboxylic acid. Nitriles can be hydrolyzed to carboxylic acids in acidic aqueous solutions, and to carboxylate salts with base-catalyzed hydrolysis:


 0 kommentar(er)
0 kommentar(er)
